- Published 23 Apr 2023
- Last Modified 17 Feb 2026
- 5 min
What is Buffer Solution?
Find out all you need to know about buffer solution.

Reviewed by Jay Proctor, Technical Support Team Leader (January 2023)
Buffer solutions, also known as pH buffers, are water-based liquids that can maintain a set pH value within a certain range when small amounts of acid or base (alkaline) are added. Made using conjugates of weak bases and acids, they can work in equilibrium together. They won’t have their pH value significantly affected when small amounts of other acids or bases are added.
Helpful in chemical and biological testing, product development and industrial processes, understanding the pH range (buffer) of each solution means its users will know where the capacity or limit will be reached and keep their conditions stable. Depending on the specific needs of a process, test or reaction, a user may need a buffer solution with a slightly acidic or alkaline pH value.
This guide will give an overview of the types of buffer solutions. It will explain how and why they’re used and how you can find the right buffer solution for your needs.
Types of Buffer Solutions
There are two main types of buffer solutions - acidic buffers and base buffers.
Acidic buffers have a pH value below 7. They can resist changes in pH when small amounts of base are added to them. For example, a buffer solution with a pH of 4 will remain around a pH of 4 even when a small amount of base is added to it.
Base buffers, also known as alkaline buffers, have a pH value above 7. They can resist changes in pH when small amounts of acid are added to them. For example, a buffer solution with a pH of 10 will remain around a pH of 10 even when a small amount of acid is added to it.
Both acidic and base buffers have a certain capacity, known as the buffer capacity, that determines how much acid or base they can neutralise before the pH of the solution starts to change significantly. The buffer capacity can be increased by adding more buffer components to the solution.
Browse Buffer Solution
Shop From Our Selection of Buffer Solutions:
Why is a Buffer Solution Used?

Buffer solutions are used for their ability to maintain a relatively constant pH in the presence of small amounts of acid or base. This property is known as buffering capacity, and it is due to the presence of weak acid and its corresponding conjugate base, or a weak base and its corresponding conjugate acid, in the solution.
When a small amount of acid is added to a buffer solution, the acid will react with the conjugate base present in the solution to form the weak acid, thus preventing a significant change in pH. Similarly, when a small amount of base is added to a buffer solution, the base will react with the conjugate acid present in the solution to form the weak acid, thus preventing a significant change in pH.
This behaviour can be explained by La Chatelier’s principle, which states that when a system at equilibrium is subjected to a change in conditions, the system will shift in such a way as to counteract the change and re-establish equilibrium. In the case of a buffer solution, the system is the equilibrium between the weak acid and its conjugate base or the weak base and its conjugate acid, and the change in conditions is the addition of acid or base. The system will shift in such a way as to neutralise the added acid or base and re-establish equilibrium, thus preventing a significant change in pH.
What is a Buffer Solution Used for?
Depending on the requirements of each process, reaction or product, acid or alkaline buffer solutions can be selected depending on their pH value. The most common uses for different buffer solutions include:
- Industrial fermentation - to convert sugar into alcohol, the pH must be kept at a specific level, which is why buffer solutions are added before fermentation
- Fabric dyeing or processing - to keep fabric dyes at an optimum pH level and ensure they work effectively, buffer solutions are used, as they are in tanning leather
- Cosmetic product development - to ensure they don’t cause skin irritation, buffer solutions stop cosmetics or hygiene products from becoming too acidic or alkaline
- Chemical analysis and research - buffer solutions are used in laboratories to ensure reactions are conducted within a specific pH range
- Medicine creation - certain biological components only operate within specific pH ranges, including enzymes, cells and blood, making buffer solutions useful in the creation of many medicines
How to Calculate the pH of a Buffer Solution
The pH levels of buffer solutions can be measured in two main ways, by using a pH meter or by calculating the Henderson-Hasselbalch equation of the solution. pH meters are instruments that measure the voltage between two electrodes to work out the level of hydrogen ions in a solution and therefore output a pH value. An objective measurement, these instruments are used in various applications, including in industry, laboratories and harsh environments.
The Henderson-Hasselbalch equation can be used to estimate or predict a solution's pH level. This equation is written as:
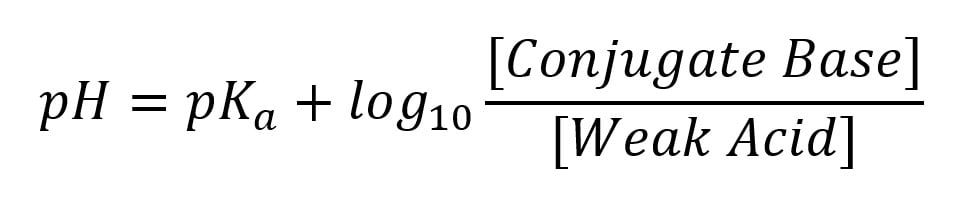
Within this equation, the pKa refers to the strength of the acid used in the solution. Although this calculation won’t always produce a completely accurate pH value, it can be useful for estimating buffer ranges or solution values before they’re created.
Further Reading
Related links
- Buffer Solutions
- Hanna Instruments HI7009L pH Buffer Solution 9.18
- Hanna Instruments HI70004P pH Buffer Solution 4.01
- Hanna Instruments HI70007C pH Buffer Solution 7.01
- Hanna Instruments HI70007P pH Buffer Solution 7.01
- Hanna Instruments HI70004C pH Buffer Solution 4.01
- Burkert 418543 pH Buffer Solution 10.01
- Burkert 418540 pH Buffer Solution 4.01







